Content Status
Type
Linked Node
Shorter oral Bedaquiline-containing MDR/RR-TB regimen
Learning ObjectivesLearn about Shorter oral Bedaquiline-containing MDR/RR-TB regimen
Based on the World Health Organization (WHO) treatment guidelines, 2020 recommendations, the National TB Elimination Programme (NTEP) have decided to transition from the current shorter injectable-containing Multi-drug Resistant (MDR)/ Rifampicin-resistant TB (RR-TB) regimen to the shorter oral bedaquiline-containing MDR/RR-TB regimen in the year 2021.
Salient Features of the Shorter Oral Bedaquiline-containing MDR/RR-TB Regimen
- This regimen will be used in patients >5 years of age weighing 15 kg or more.
- The regimen will be expanded in a phased manner starting with selected states to gain programmatic experience to guide future expansion within 2021.
- The regimen consists of an initial phase of 4 months that may be extended up to 6 months and a continuation phase of 5 months, giving a total duration of 9-11 months. Bdq is used for a duration of 6 months.
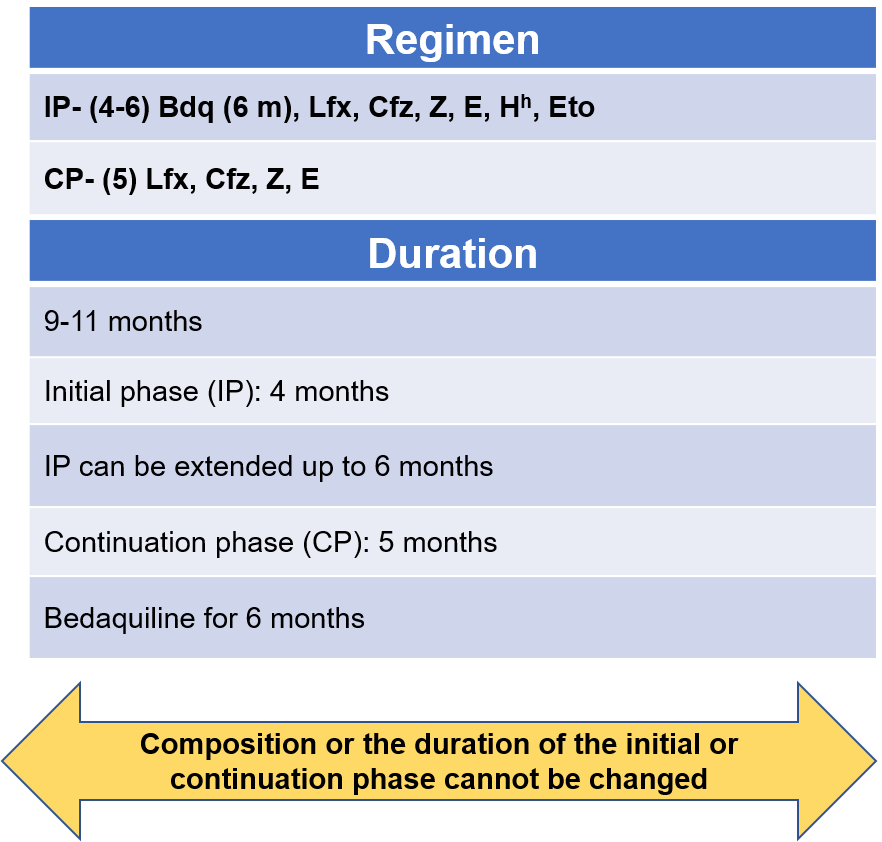
Figure 1: Regimen and duration of shorter oral Bdq-containing MDR/RR-TB regimen
Abbr: Bdq - Bedaquiline, Lfx- Levofloxacin, Cfz- Clofazamine, Z- Pyrazinamide, E- Ethambutol, Hh- High-dose Isoniazid, Eto- Ethionamide
Points to Note
- From the start to the end of 4 months these drugs are used: Bedaquiline, Levofloxacine, Clofazamine, Pyrazinamide, Ethambutol, high-dose Isoniazid, Ethionamide
- From the start of 5 months to the end of 6 months (If IP not extended): Bdq, Lfx, Cfz, Z, E
- From the start of 7 months to the end of 9 months: Lfx, Cfz, Z, E
If the IP is extended up to 6 months, then all 3 drugs Bdq, Hh and Eto are stopped together
Resources
- Guidelines for Programmatic Management of Drug-resistant Tuberculosis in India, March 2021.
- WHO Consolidated Guidelines on Tuberculosis, Module 4 - Treatment: Drug Resistant TB Treatment.
Kindly provide your valuable feedback on the page to the link provided HERE
Content Creator
Reviewer
Target Audience
- Log in to post comments