Content Status
Type
Linked Node
Basics of Stock management
Learning ObjectivesStock management in NTEP
Stock management refers to the activities undertaken to ensure sufficient stock of drugs/ items at all stocking points. It is facilitated through Ni-kshay Aushadhi.
The stock management process under the National TB Elimination Programme (NTEP) includes the following activities:
-
Drug Inventory
-
Physical Stock Verification
-
Expiry Management
1) Drug Inventory:
Drug inventory is the module available under Ni-kshay Aushadhi used to manage the inventory/ storage of drugs available at the store. Certain drug inventory activities like determination of drug stock status at the State Drug Stores (SDS), District TB Centres (DTC)/ Tuberculosis Unit (TU)/ Peripheral Health Institution (PHI), correction of imbalances through transfers, replenishment and requisitioning of stocks are carried out by the officer-in-charge of the logistics function at the State TB Office (STO).
Ni-kshay Aushadhi displays a Drug Inventory Desk that provides the following information to the users:
-
Drugs available at the store: The information about the drug, its stock quantity with units of measure and expiry is shown in the table.
-
Near-expiry drugs: The system shows the near-expiry drugs in ‘Grey’ colour.
-
Expired stock: Expired drugs are shown in ‘Pink’ colour.
-
Incomplete boxes: Boxes which are made incomplete are shown in ‘Blue’ colour.
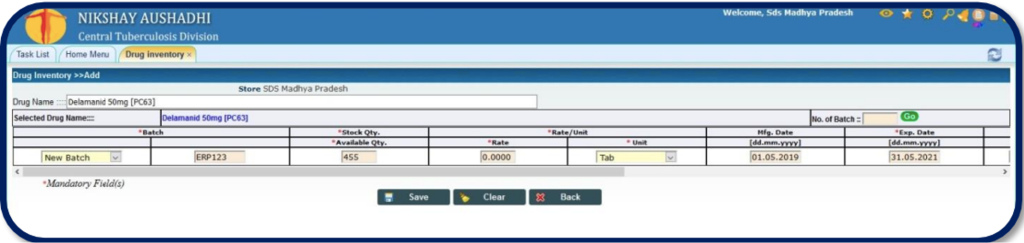
Figure 1: Drug Inventory Desk on Ni-kshay Aushadhi; Source: Drug Inventory and Physical Stock Verification, Ni-kshay Aushadhi User Manual, CTD, MoHFW, India.
2) Physical Stock Verification (PSV):
It comprises a set of procedures to be followed for the physical verification and reconciliation of anti-TB drug stocks at the drug stores. PSV should be carried out under the supervision of the concerned officer-in-charge at the state, DTC, TU and PHI drug stores at the end of each month/year and also surprise checks in between.
On the last working day of each month, the storekeeper shall verify the number of cartons/ boxes/ strips physically available at the store for each of the drugs dealt with by the programme and cross-check it with the number of drugs that should have been actually available. All details are recorded on Ni-kshay Aushadhi.
Reconciliation to address any discrepancies should be undertaken by verifying PSV with the stock register. The concerned officer-in-charge shall review and sign off the PSV after thorough verification and validation of all receipts and issues that have been recorded in the stock register; verification of consistent recording of batch numbers, date of manufacture and date of expiry of drugs in the stock register at the time of receipt of each consignment; compliance to First Expiry First Out (FEFO) principle; etc. Any un-reconciled discrepancies determined through the above process should be reported to the STO and Central TB Division (CTD).
The ‘Physical Stock Verification’ under Ni-kshay Aushadhi process is a two-step process.
-
In the first step, user can change the quantity of the existing batch/ drug or add a new batch/ drug and click on the ‘Final Save’ button.
-
Then in the second step the user can view (V), modify (M), cancel (C) or final save/ upload (S) the stock and generate the voucher.
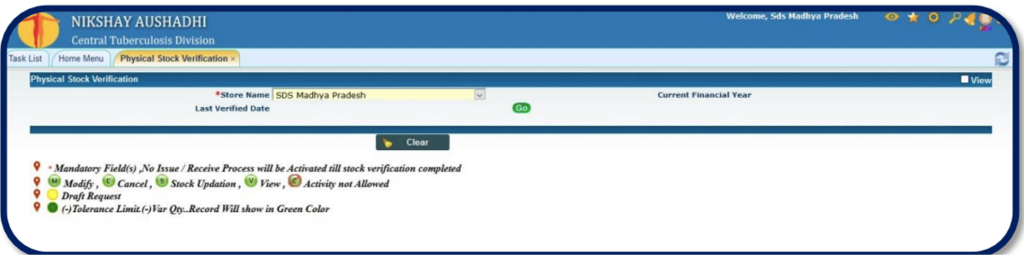
Figure 2: Physical Stock Verification Desk; Source: Drug Inventory and Physical Stock Verification, Ni-kshay Aushadhi User Manual, CTD, MoHFW, India.
3) Expiry Management:
Refers to a set of procedures to be followed for the management of short expiry drugs and immediate steps in dealing with the same so as to ensure their utilisation within their shelf-life.
The storekeeper is expected to incorporate appropriate tools to periodically monitor controls over the expiry position of drugs held in stocks mainly through storage of drugs of a particular description at one place, expiry-wise stacking and marking expiry dates on cartons/ drug boxes with marker pens. The storekeeper shall strictly follow FEFO principles while issuing drugs at all times.
If any drug expires due to reasons beyond control, the write-off of expired drugs should be as per the guidelines given in NTEP National Strategic Plan (NSP) - 2017. As per the NSP, the state is allowed to write off up to 2% of the cost of the annual supply of drugs on implementation of DST-guided treatment and 2% cost of rapid molecular test cartridges. The expired stock should be disposed of as per the Biomedical Waste (BMW) Management and Handling Guidelines of the Government of India.
Resources
-
Standard Operating Procedure Manual Procurement & Supply Chain Management, CTD, MoHFW, India, 2018.
-
Drug Inventory and Physical Stock Verification, Ni-kshay Aushadhi User Manual, CTD, MoHFW, India.
Assessment
|
Question |
Answer 1 |
Answer 2 |
Answer 3 |
Answer 4 |
Correct answer |
Correct explanation |
Page id |
Part of Pre-test |
Part of Post-test |
|
On Ni-kshay Aushadhi, drug stock inventory can be viewed through which desk? |
Expiry Management Desk |
Drug Inventory Desk |
Physical Drug Verification Desk |
None of the above |
2 |
On Ni-kshay Aushadhi, drug stock inventory can be viewed through the Drug Inventory Desk. |
|
Yes |
Yes |
Content Creator
Reviewer
- Log in to post comments