Content Status
Type
Linked Node
Treatment Algorithm for MDR/RR-TB
Learning Objectives- Understand the treatment algorithm for multidrug-resistant (MDR) and rifampicin-resistant (RR) tuberculosis (TB) cases.
- Identify the sequential steps involved in the management of MDR/RR-TB, including drug selection, regimen design, and treatment duration.
- Identify criteria for exclusion.
The treatment algorithm for Multidrug-resistant/ Rifampicin-resistant TB (MDR/ RR-TB) patients is a part of an integrated diagnostic and treatment algorithm under Programmatic Management of Drug-resistant Tuberculosis (PMDT).
A shorter oral Bedaquiline (Bdq)-containing MDR/ RR-TB regimen is recommended for those MDR/ RR-TB patients in whom resistance to the component drugs has been excluded or those who have not been previously treated for more than one month with second-line drugs used in shorter oral Bdq - containing MDR/ RR-TB regimen and have no other exclusion criteria.
All those MDR/ RR-TB patients who are not eligible for a shorter Bdq - containing regimen, after careful evaluation, are considered for a longer M/ XDR-TB regimen.
The treatment algorithm for MDR/ RR-TB patients is shown below.
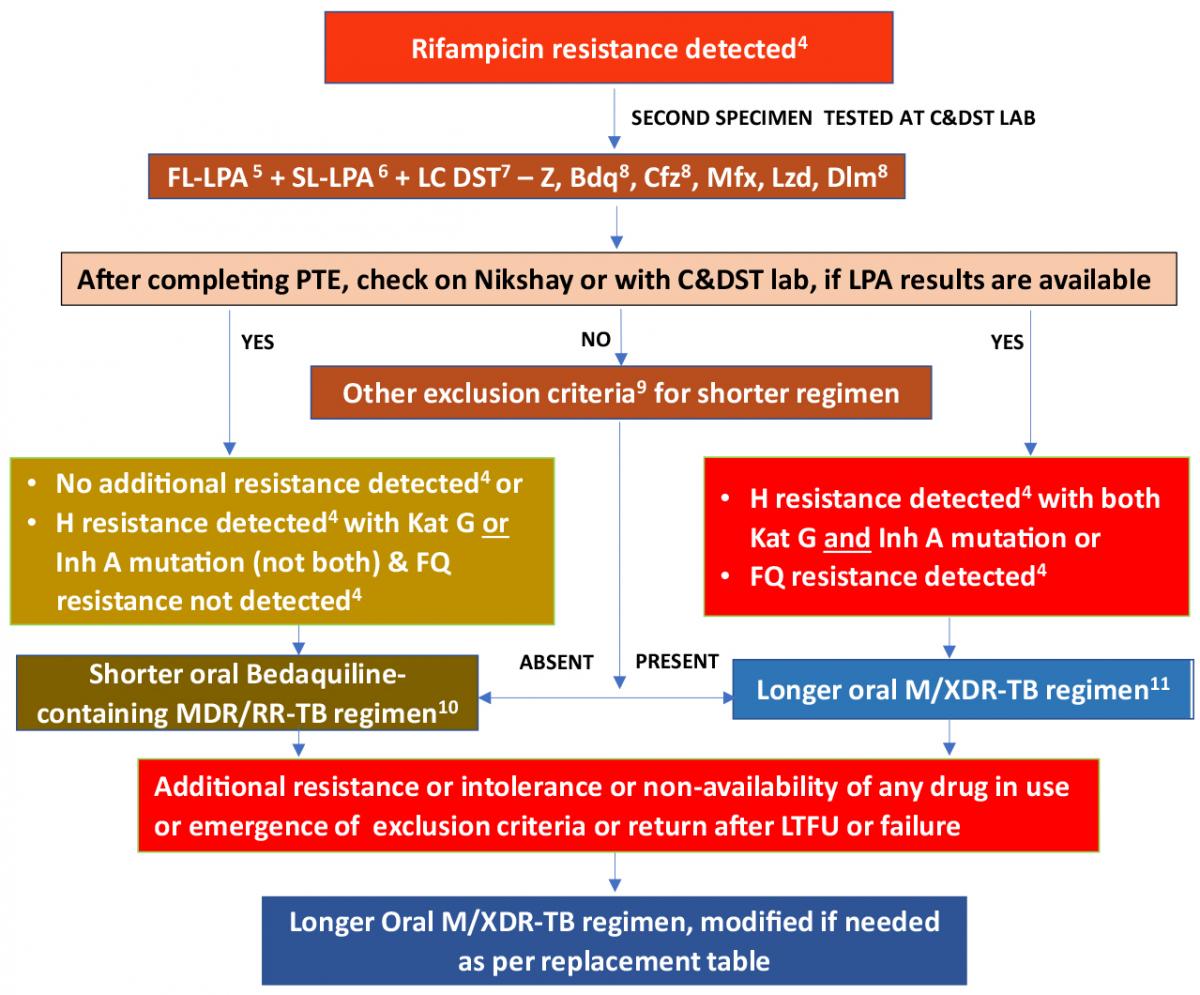
Figure: Treatment Algorithm for MDR/RR-TB; Source: PMDT Guidelines India, March 2021, p46.
Footnotes for the Algorithm
4 As per mutation pattern, includes resistance inferred.
5 Discordance in RR results between Nucleic Acid Amplification Tests (NAAT) & First-line Line Probe Assay (FL-LPA) to be resolved with a repeat NAAT at Culture and Drug Susceptibility Testing (C&DST) lab and microbiologists will provide the final decision. InhA mutation is associated with Eto resistance. Use other exclusion criteria to decide regimen if FL-LPA is done on culture isolates for patients with smear-negative specimens.
6 To assess Levofloxacin (Lfx), Moxifloxacin (Mfx) and Amikacin (Am) resistance.
7 Start treatment based on Line Probe Assay (LPA) results and modify based on Liquid Culture (LC) and DST results later.
8 Whenever DST is available.
9 Other exclusion criteria for shorter oral Bdq-containing MDR/ RR-TB regimen includes:
- History of exposure for > 1 month to Bdq, Lfx, Ethionamide (Eto), or Clofazimine (Cfz), if the result for DST (Bdq, Fluoroquinolone (FQ), Inh A mutation, Cfz & Pyrazinamide (Z)) is not available.
- Intolerance to any drug or risk of toxicity from a drug in the shorter oral Bdq-containing MDR/ RR-TB regimen (e.g. drug-drug interactions).
- Extensive TB disease – the presence of bilateral cavitary disease or extensive parenchymal damage on chest radiography. In children aged under 15 years, presence of cavities or bilateral disease on chest radiography.
- Severe Extrapulmonary TB (EP-TB) disease - the presence of miliary TB or TB meningitis or Central Nervous System (CNS) TB. In children aged under 15 years, extra-pulmonary forms of disease other than lymphadenopathy (peripheral nodes or isolated mediastinal mass without compression).
- Pregnant and lactating women (with conditional exceptions).
- Children below 5 years.
10 This portion applies as states move to a shorter oral Bdq-containing MDR/ RR-TB regimen under the guidance of the National TB Elimination Programme (NTEP).
11 Patients who were on a longer oral M/ XDR-TB regimen based on the history of exposure for >1 month and in whom resistance is not detected to Isoniazid (H) or FQ may be switched to shorter oral Bedaquiline containing MDR/ RR-TB regimen based on the FL and Second-line LPA (SL-LPA) results if the duration of longer oral M/ XDR-TB regimen drugs consumed is <1 month.
Resources
- Guidelines for Programmatic Management of Drug-resistant Tuberculosis in India, 2021.
- WHO Consolidated Guidelines on Tuberculosis: Module 4 – Treatment: Drug-resistant TB Treatment, 2020.
Kindly provide your valuable feedback on the page to the link provided HERE
Page Tags
Content Creator
Reviewer
Target Audience
- Log in to post comments