Content Status
Type
Linked Node
Line Probe Assay [LPA]
Learning Objectives- Provide an overview of the LPA Test- A rapid molecular test available at centralized laboratories using a combination of PCR, Gel Electrophoresis technologies. Primarily used for the diagnosis of DRTB.
- Discuss the important advantages and disadvantages of the platform.
- Rapid test
- Multiple tests at a time-Larger throughput
- lower sensitivity requiring culture
- Many genetic probes tested simultaneously
- Provide a brief overview(pictures) of the LPA laboratory and processing.
H5Content
Content
Line Probe Assay (LPA) is a rapid molecular test available at centralised laboratories.
The assay is based on Polymerase Chain Reaction (PCR) that can simultaneously detect Mycobacterium tuberculosis complex as well as drug sensitivity to anti-TB drugs.
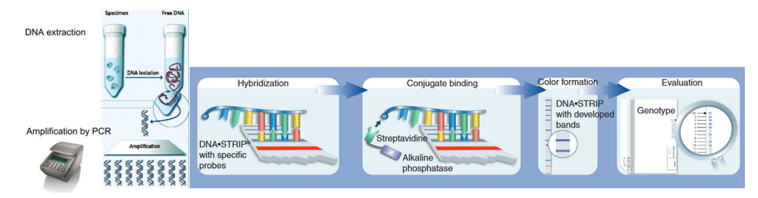
Figure 1: The GenoType MTBDRplus Molecular LPA Procedure; Source: Molecular Detection of Drug-resistant Tuberculosis by Line Probe Assay.
Advantages of LPA
- Rapid molecular test. (Turnaround time: 3-5 days)
- Highly sensitive and specific.
- Performed directly from sputum bacteriologically positive specimens and on isolates of M. tuberculosis complex grown from bacteriologically negative and bacteriologically positive specimens.
- Detects multiple gene mutations in anti-TB drugs.
- First-line LPA detects mutations to rifampicin and isoniazid
- Second-line LPA detects mutations to fluoroquinolones and aminoglycosides.
- Suitable for low and high-throughput labs.
Disadvantages of LPA
- Cannot be used as a point-of-care test.
- Requires appropriate laboratory infrastructure, equipment and biosafety precautions.
- Different rooms (DNA extraction, pre-amplification, amplification, post-amplification/ hybridization) are required to perform different steps (Figure 2).
- Requires trained manpower to perform tests and interpret test results.
- Stringent internal quality control is required to prevent contamination.

Figure 2: Amplification (A) and Post-amplification Laboratory (B) for LPA; Source: Molecular Detection of Drug-resistant Tuberculosis by Line Probe Assay.
Resources
- Guidelines for PMDT in India, 2021.
- Molecular Detection of Drug-resistant Tuberculosis by Line Probe Assay.
Assessment
| Question | Answer 1 | Answer 2 | Answer 3 | Answer 4 | Correct answer | Correct explanation | Page id | Part of Pre-test | Part of Post-test |
| LPA can be used as a point-of-care test. | True | False | 2 | LPA cannot be used as a point-of-care test. | | Yes | Yes |
LMS Page Link
Content Creator
Reviewer
Target Audience
- Log in to post comments