Content Status
Type
Linked Node
Stock Register at a Microscopy Centre
Learning ObjectivesThe learner will be able to
- Discuss the details of stock register maintained in DMC
- Explain responsibiliyies for maintenance of stock register and
- Recall calculation of stocks required at DMC
- Designated Microscopy Centres (DMCs) are the most peripheral laboratory under the National TB Elimination Programme (NTEP) network. Therefore, it is very important for the DMCs to maintain an adequate stock of all consumables.
- A paper-based stock register is maintained at the DMC and submitted as a part of the ‘Monthly report on programme management, logistics and microscopy’.
- All the DMCs as well as Peripheral Health Institute (PHI) that are a DMC need to fill the second part of this monthly report format for reporting the status of laboratory consumables and equipment (Figure below).
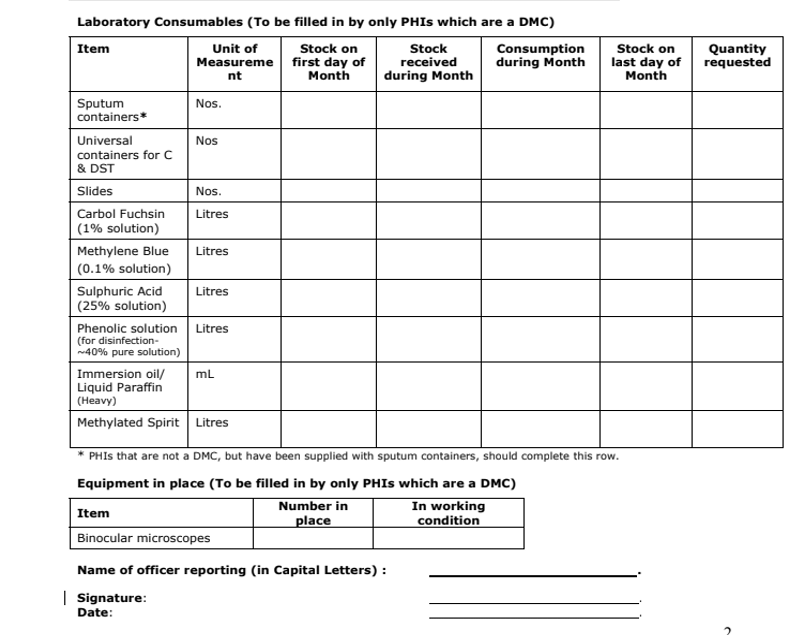
Figure: Monthly report format for reporting status of laboratory consumables and equipment, to be filled by PHI that is DMC.
- The stock register for consumables at the DMC has the provision to enter the information about the stock of consumables that are available at the DMC on the first day of the month, stock received and consumed during the month and stock remaining on the last day of the month along with the requested quantity of new stock.
- The Lab Technician (LT) of the DMC is responsible for exhausting the old supplies before the new ones.
- The Medical Officer (MO) of the DMC is responsible for determining the stocks and the Senior Tuberculosis Lab Supervisor (STLS) should ensure these supplies are distributed in a timely manner, as and when required.
Table: Calculation of Stocks Required at the DMC
| Sputum containers |
For diagnosis:
|
|
For follow-up:
|
|
| Slides |
|
| Reagents |
|
|
CBNAAT/ Truenat Machines and Cartridges/ Chips
|
|
| Binocular Microscopes (BM) and LED Fluorescence Microscopes (FM) |
|
| Tuberculosis Laboratory Register |
|
| Laboratory Form for Sputum Examination |
|
Resources
- Training Modules (1-4) for Programme Managers and Medical Officers, CTD, MoHFW, GoI, 2020.
- Module for STS Part 2: Ensuring Proper Registration and Reporting. CTD, MoHFW, India.
Assessment
| Question | Answer 1 | Answer 2 | Answer 3 | Answer 4 | Correct answer | Correct explanation | Page id | Part of Pre-test | Part of Post-test |
| How many TB Laboratory Register/s is/are required in one year for each DMC? | 1 | 2 | 3 | 4 | 1 | Each Tuberculosis Laboratory Register allows for the registration of at least 2000 patients. For each lakh, 75 smear-positive patients are projected, requiring the examination of 750 patients (thrice each). Additional follow-up examinations will bring the number of registers needed to approximately one lab register/ DMC. | | Yes | Yes |
|
For follow-up, approximately 0.2 laboratory forms for sputum examination are needed for each pulmonary tuberculosis case. |
True | False | 1 |
For diagnosis, approximately 10 laboratory forms for sputum examination are needed. 10 is the average number of symptomatic for each case of pulmonary smear-positive tuberculosis identified. 1 out of 10 examined will be smear-positive, each needs two forms for follow-up. When calculated, out of 10 it will be 0.2 |
Yes |
Yes |
Content Creator
Reviewer
Target Audience
- Log in to post comments