Content Status
Type
Linked Node
Reporting of ICF activities among PLHIV
Learning ObjectivesIntensified TB case finding at ICTCs/LAC/ART centres/LAC Plus centre
Recording and Reporting of Intensive Case Finding (ICF) among People Living with HIV (PLHIV)
Reporting of ICF is an important activity for coordination between NACP and NTEP. Since ICF activities occur at the ICTCs, LAC and ART centres, the recording and reporting of the same should be done to the NTEP using line lists and consolidated reports. The details about referrals have to be filled by the ART staff (counsellor/nurse), and details about TB diagnosis and treatment initiation have to be filled by the NTEP staff (STS). These records are validated during monthly HIV/TB coordination meetings. It helps to maintain continuum of care between the two programs.
Integrated Counselling and Treatment Centres (ICTCs) and Link Antiretroviral Treatment (ART) Centre (LAC)
In all ICTCs and LAC (because ICTC counsellor operated the LAC), referrals of TB suspects should be recorded on the ICTC line list to facilitate coordination with National TB Elimination Programme (NTEP) to determine TB diagnosis and initiation of DOTS of the referred patients.
To streamline this process further NTEP staff should stay in touch with ICTC counsellors to complete the exchange of information in time.
It is crucial that the ICTC counsellor attends the NTEP monthly meeting for coordination with NTEP staff to validate the line lists, and monthly HIV/ TB reports and solve operational issues if any.
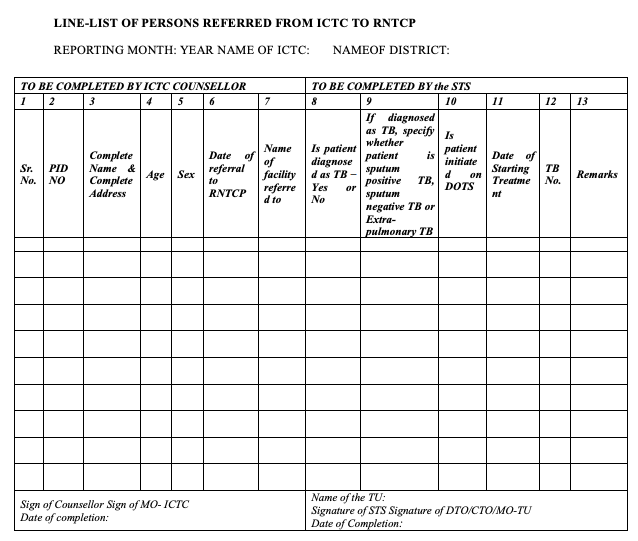
Figure 1: Line-list of persons referred from ICTC to NTEP; Source: National Framework for Joint HIV-TB Collaborative Activities, 2013.
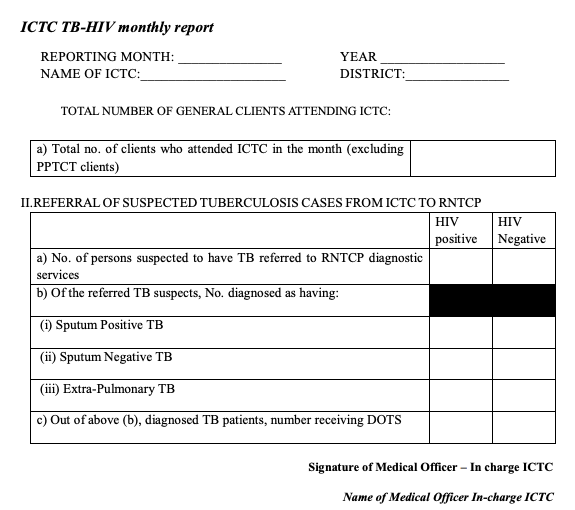
Figure 2: ICTC TB-HIV Monthly Report; Source: National Framework for Joint HIV-TB Collaborative Activities, 2013.
ART Centres/ LAC Plus
All referrals of presumptive TB cases from ART Centre/ LAC plus centres should be recorded on an ART centre TB-HIV line list to facilitate coordination with NTEP programme staff and to track the patient closely through the process of TB diagnosis and DOTS initiation. It is also crucial that ART centre staff members attend monthly HIV/TB coordination meetings.

Figure 3: Line-list of persons referred from ART centre to NTEP; Source: National Framework for Joint HIV-TB Collaborative Activities, 2013.
The HIV/TB monthly reporting format generated at ART centres is incorporated into the ART centre monthly report (CMIS).
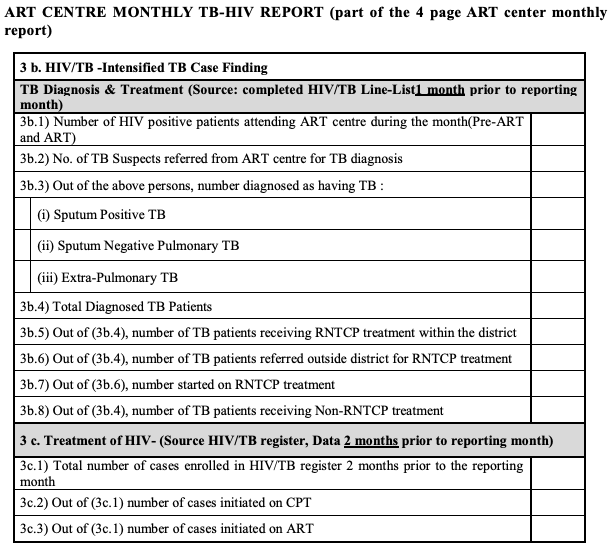
Figure 4: ART Centre Monthly TB-HIV Report; Source: National Framework for Joint HIV-TB Collaborative Activities, 2013.
Information about all HIV-infected TB patients in HIV care should be recorded in the ART centre's HIV/TB register. These include:
- TB patients detected by ART/ LAC plus centre staff
- TB patients found HIV-infected while on DOTS treatment and referred to ART centre by the RNTCP
TB-HIV register is an important monitoring tool to track the timeliness of initiation of Cotrimoxazole Preventive Therapy (CPT) and ART, and also the TB treatment outcome so as to modify Antiretroviral (ARV) regimens as per guidelines.
It is important that ART centre staff carry this register when they attend monthly HIV/TB coordination meetings to update information on TB treatment outcomes from NTEP staff and share information pertaining to CPT and ART with them for recording into NTEP TB registers.
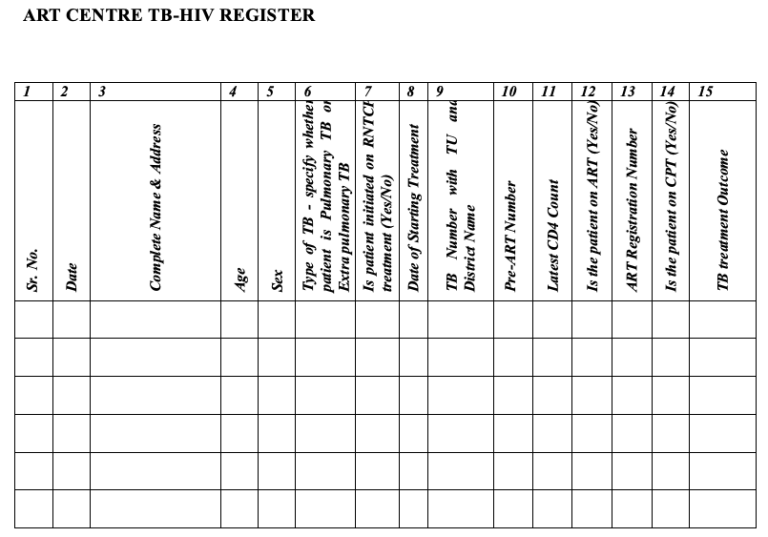
Figure 5: ART Centre TB-HIV Register; Source: National Framework for Joint HIV-TB Collaborative Activities, 2013.
References
- National Framework for Joint HIV-TB Collaborative Activities, Department of AIDS Control, CTD, MoHFW, GoI, 2013.
- Operational Guidelines for ART Services, NACO, 2012.
Assessment
|
Question |
Answer 1 |
Answer 2 |
Answer 3 |
Answer 4 |
Correct Answer |
Explanation |
Page ID |
Part of Pre-test |
Part of Post-test |
|
Which of the following tracks the status of ART, ATT and CPT? |
ICTC line list for presumptive TB |
ART centre TB-HIV monthly report |
TB - HIV register |
ART centre TB -HIV line list |
3 |
TB-HIV register is an important monitoring tool to track the timeliness of initiation of CPT and ART also the TB treatment outcome so as to modify ARV regimens as per guidelines. |
|
yes |
yes |
Content Creator
Reviewer
Target Audience
- Log in to post comments