Content Status
Type
Linked Node
Laboratory findings of NTM
Learning ObjectivesLaboratory findings of NTM
AFB Smear and NAAT Test (LPA/CBNAAT/TRUENAT)
- Two specimens are to be collected: One must be a morning specimen and sent for AFB Smear. Induction of sputum with hypertonic saline may be used in patients who are unable to produce sputum spontaneously.
- If a smear-positive (AFB-positive) specimen shows results for M.TB but is not detected by NAAT technology, the specimen should be inoculated for LC/LJ culture to check the presence of NTM.
- NAAT (LPA/CBNAAT/TRUENAT) detect only M. TB Complex ……..NOT NTM.
Culture Method for NTM Diagnosis
- Culture remains the gold standard for laboratory confirmation of NTM and is required for genotypic identification and drug susceptibility tests (DST).
- The culture media are similar to those used for M. tuberculosis.
- Solid media include either egg-based media, such as Löwenstein-Jensen agar, or agar-based media, such as Middlebrook 7H10 and 7H11 media.
-
Liquid systems are more sensitive and reduce the delay in the detection of NTM, but they are prone to contamination by other microorganisms and bacterial overgrowth. In the laboratory, an incubator system of liquid culture, which contains enriched Middlebrook 7H9 broth, can automatically detect the growth of mycobacteria, including NTM.
NTM identification on Culture
>7 Days Slow Growers NTMs
|
Type-I True Pathogens (Photochromogens)
|
Type-II Saprophytes (Scotochromogens) • Mycobacterium gordonae • Mycobacterium scrofulaceum |
Type-III Opportunistic Pathogens (Non-chromogens) • Mycobacterium avium complex • Mycobacterium ulcerans • Mycolicibacter terrae |
<7 Days Rapid Growers NTMs
| Type-IV Opportunistic Pathogens (Non-chromogens) • Mycolicibacterium fortuitum • Mycobacteroides abscessus • Mycobacteroides chelonae |
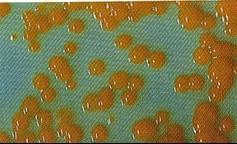
Appearance of Atypical / NTMs Colony Morphologies (Pigmented & Non-Pigmented)
| Atypical / NTMs Colony Morphologies (Pigmented & Non-Pigmented) |
Image

|
Donut Colonies of M.fortuitum on egg medium |
|
Image

|
Smooth dome shaped Colonies of M.chelonae on egg medium | |
|
Image
|
Smooth dome shaped Colonies of M.szulgai on egg medium | |
|
Image

|
Smooth dome shaped Colonies of M. scrofulaceum on egg medium | |
|
Image

|
Orange Colored Smooth dome shaped Colonies of M.gordonae on egg medium | |
|
Image

|
Rough colonies of M. triviale on egg medium | |
| Adopted from: Kent TP and Kubica GP., Public Health Mycobacteriology. A Guide for Level III Laboratory, Vol.30, United States Department of Health and Human Services, Centre for Disease Control, Atlanta, USA, 1985. | ||
Drug susceptibility test for NTM
- The role of a DST is to guide the design of optimal treatment regimens. However, the DST for NTM is difficult and controversial because of discrepancies between in vitro and in vivo clinical outcomes, with the exception of macrolides and amikacin.
- Among slow-growing mycobacteria (SGM), clear correlations have been established for macrolides and amikacin in MAC lung disease and for rifampicin in M. kansasi lung disease.
- Macrolide resistance in MAC is caused by a mutation in the 23S rRNA gene macrolide binding site, usually selected due to macrolide monotherapy.
- Only routine macrolide susceptibility testing for all MAC isolates is advised, and in the case of macrolide resistance, moxifloxacin and linezolid should be tested.
Content Creator
Reviewer
- Log in to post comments