Content Status
Type
Linked Node
Procedures following the receipt of supplies
Learning Objectives6 Procedures to be followed
H5Content
Content
The State Drug Store (SDS) pharmacist should follow certain procedures after receipt of supplies.
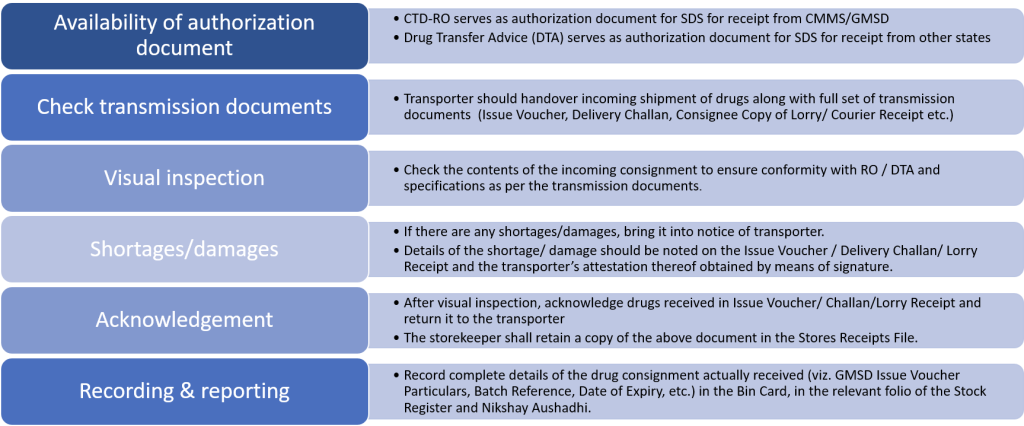
Figure 1: Overview of procedures following receipt of supplies by a State Drug Store (SDS)
Visual Inspection :
- The check shall be limited to visual inspection and count of the number of cartons received and matching the same with the Issue Voucher and Challan. The Storekeeper will not ordinarily open sealed cartons unless the seal and/or exterior suggest damage or shortage or there have been frequent shortages observed in the recent past.
- In case, where the GMSD/CMSS has opted to make part shipments, the Storekeeper shall record details of drugs received and the balance quantity pending supply. The Storekeeper shall follow-up closely with the supplier.
Shortages and/ or transit damages:
- In the case of shortage/ damage determined by the Storekeeper through visual inspection s/he shall take the precaution of opening the seals of all cartons received and carefully checking their contents down to the lowest packaging unit.
- Ideally, SDS should take custody only of undamaged stock from the perspective of the drugs in question being in a good enough condition to be administered to patients. SDS storekeeper shall segregate and preserve damaged stocks till further instructions are received.
Recording and Reporting
- In the case of shortage/ damage/ discrepancy in the quantity of drugs actually received vis-à-vis that indicated as per the transmission/ authorization document, record complete details of the same in the ‘Remarks’ column of the SR & NA and highlight the same.
- In case transmission documents are received prior to receipt of drugs, entry shall not be made in the SR on the basis of such documents viz. RO, Issue Voucher / Challan.
- Alternatively, if drugs are received prior to receipt of transmission documents, entry in SR shall be made only after their receipt and confirmation as to the quantity supplied by respective sending unit.)
Resources
Assessment
| Question | Answer 1 | Answer 2 | Answer 3 | Answer 4 | Correct answer | Correct explanation | Page id | Part of Pre-test | Part of Post-test |
| Which document should be received before or along with the consignment from GMSDs? | State Issue Voucher (SIV) | CTD-Release Order (CTD-RO) | District Issue Voucher (DIV) | Quarterly Report on Programme Management & Logistics (QRPML) | 2 | Upon the receipt of consignment from GMSDs the pharmacist will ensure that a release order from the CTD-Release Order (CTD-RO), issued by the Central TB division is received either before or along with the consignment. | | Yes | Yes |
LMS Page Link
Content Creator
Reviewer
Target Audience
- Log in to post comments