Content Status
Type
Linked Node
What is Quality Assurance of Drugs in NTEP?
Learning ObjectivesDefinition and significance
Procurement of good quality anti-Tuberculosis (TB) drugs and ensuring the quality of drugs till the consumption point is one of the prime objectives of National TB Elimination Programme (NTEP).
To ensure the quality and efficacy of anti-TB drugs, a comprehensive NTEP Quality protocol has been developed and is being followed during the procurement mechanism and supply chain management of drugs up to the consumption points. Simultaneously, quality assurance of anti-TB drugs has also been aligned with Ni-kshay Aushadhi application for easy reporting and recording from the Central to the peripheral level.
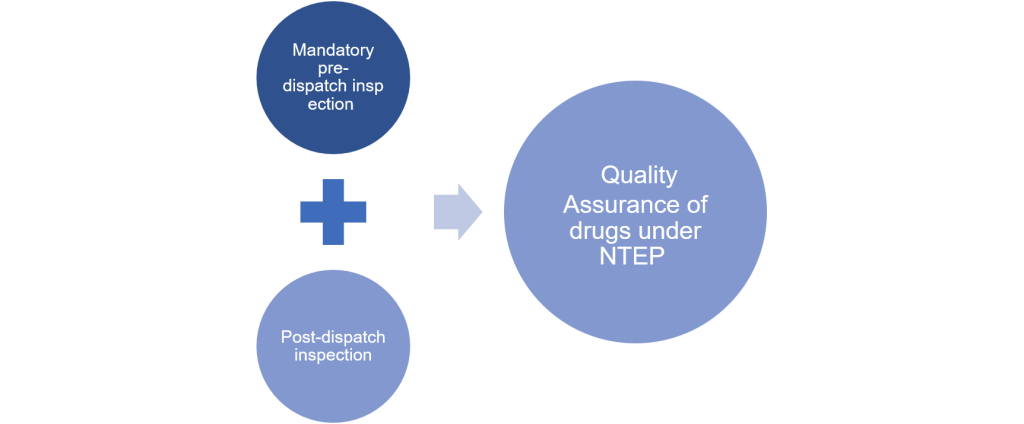
Fig 1: Quality assurance of drugs under NTEP
Resources
1. Standard Operating Procedure Manual- Procurement & Supply Chain Management; RNTCP, MoHFW, GoI 2019.
2. NTEP training modules 5-9; Central TB Division, MoHFW, GoI 2020.
Assessment:
| Question | Answer 1 | Answer 2 | Answer 3 | Answer 4 | Correct answer | Correct explanation | Page id | Part of Pre-test | Part of Post-test |
| What is included in the quality assurance of drugs under NTEP? | Pre-dispatch inspection | Post-dispatch inspection | All of the above | None of the above | 3 | NTEP protocol for quality assurance of drugs includes mandatory pre-dispatch inspection and post-dispatch inspection | Yes | Yes |
Content Creator
Reviewer
Target Audience
- Log in to post comments