Content Status
Type
Linked Node
Collection of drugs for testing
Learning Objectivesfrom where to pick, how many to pick, sent for testing/quarantine, recording, batch no to be recorded
Directions for collection of drug quantities for testing
- Central Tuberculosis (TB) Division will issue directions every quarter to concerned Government Medical Store Depots (GMSDs) and states for the collection of drug samples as per National TB Elimination Programme (NTEP) Quality Assurance (QA) protocol.
- Directions will be accompanied by the name of the drugs and the number of batches to be collected from respective drug stores.
- Generally, at least two batch numbers of drug samples selected randomly shall be sampled for testing from GMSDs, State and District Drug Stores.
- From Tuberculosis Unit (TU), only one batch number of drugs may be sampled. In case of stock shortage, samples will not be taken from TU.
Table 1: Quantities as per formulations to be collected for testing
| S.No | Formulation | Quantity for collection |
| 1. | Tablet | 10 strips |
| 2. | Capsule | 10 strips |
| 3. | Injectable | 80 vials |
| 4. | DSTB-IP / (A) 4FDC | 8 Blister Strips |
| 5. | DSTB-CP (A) / 3FDC-A | 8 Blister Strips |
| 6. | DSTB-IP (P)/ 3FDC-P | 8 Blister Strips |
| 7. | DSTB-CP (P) / 2FDC-P | 8 Blister Strips |
Procedure for collection of drug samples for testing
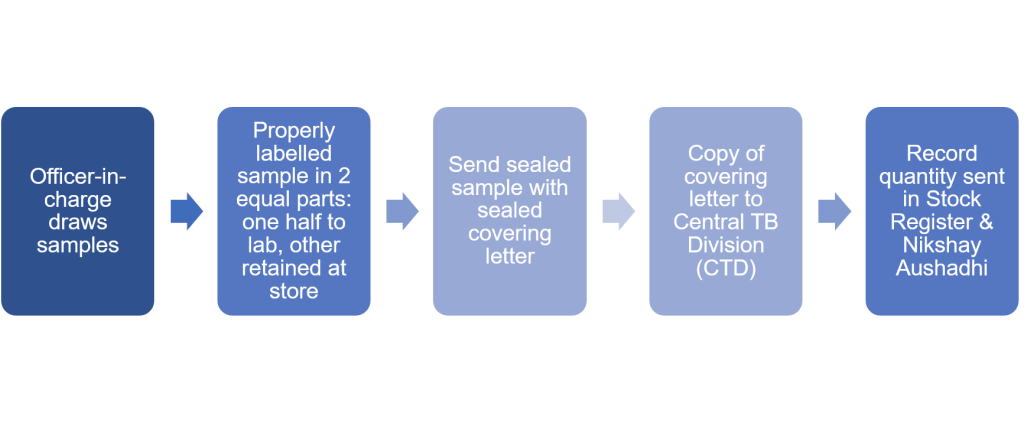
Figure 1: Overview of collection and sending of drug samples for quality testing in NTEP
The following procedure should be followed for the collection of drug samples:
1. As far as possible, the officer-in-charge should draw drug samples from original, unopened boxes /containers/ packs.
2. The sample drawn shall be divided into two equal parts, one half to be sent to the contracted laboratory in sealed condition and another half to be retained at the drug store in sealed condition.
3. The sealed pack of drugs collected should indicate on its label or otherwise:
(1) Drug name
(2) Quantity
(3) Batch No.
(4) Date of Manufacturing
(5) Date of Expiry
(6) Supplier Name
(7) Procurement Agency
(8) Manufacturing License No.
(9) Source of collection besides caution (if any) printed on the label for use/ storage of the product.
4. Information as above should be repeated in a covering letter, sealed and sent along with the sealed sample to the laboratory.
5. A copy of the covering letter should also be sent to Central TB Division.
6. Sample quantities collected should be such that the samples collected can be analyzed twice (as indicated above, by dividing into two equal batches).
7. Half of the sample collected should be sent to the selected laboratory in a sealed condition and the remaining half-sample of the same batch should be retained in sealed condition at the concerned drug stores until the lab report on the sample is received.
8. Record the quantities issued to testing laboratories in the stock register and Ni-kshay Aushadhi.
Resources
1. Standard Operating Procedure Manual-Procurement & Supply Chain Management; RNTCP, MoHFW, GoI 2019.
Assessment:
| Question | Answer 1 | Answer 2 | Answer 3 | Answer 4 | Correct answer | Correct explanation | Page id | Part of Pre-test | Part of Post-test | ||
| Drug samples collected for quality assurance should be divided into 2 equal parts. | True | False | 1 | The sample drawn shall be divided into two equal parts, one half to be sent to the contracted laboratory in sealed condition and another half to be retained at the drug store in sealed condition. |
Content Creator
Reviewer
Target Audience
- Log in to post comments